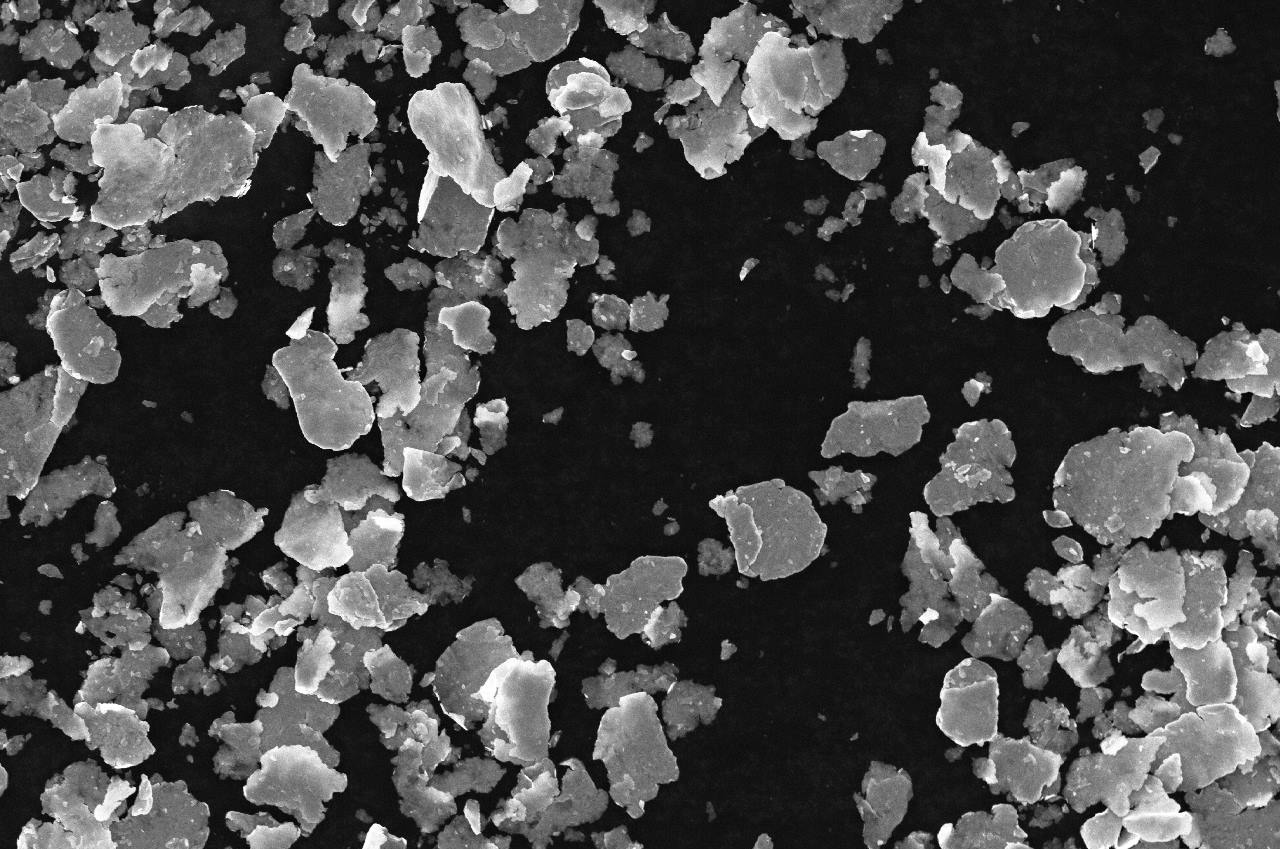
Zinc flake coatings
Bring active protection to your parts
We have invented the zinc flake coating technology to protect metal parts against corrosion at low thickness and with non-electrolytic application process. It consists of passivated zinc and aluminum flakes in an organo-mineral binder. The flake geometry increases the zinc and aluminum active surface, which allows to use less material and achieve higher anti-corrosion performances.
How it works?
Barrier effect
The positioning of the zinc flakes gives the film a barrier effect that isolates the metal from the corrosive environment. Indeed the flake morphology increases the length of the path that the aggressive substances must travel to reach and corrode the substrate.
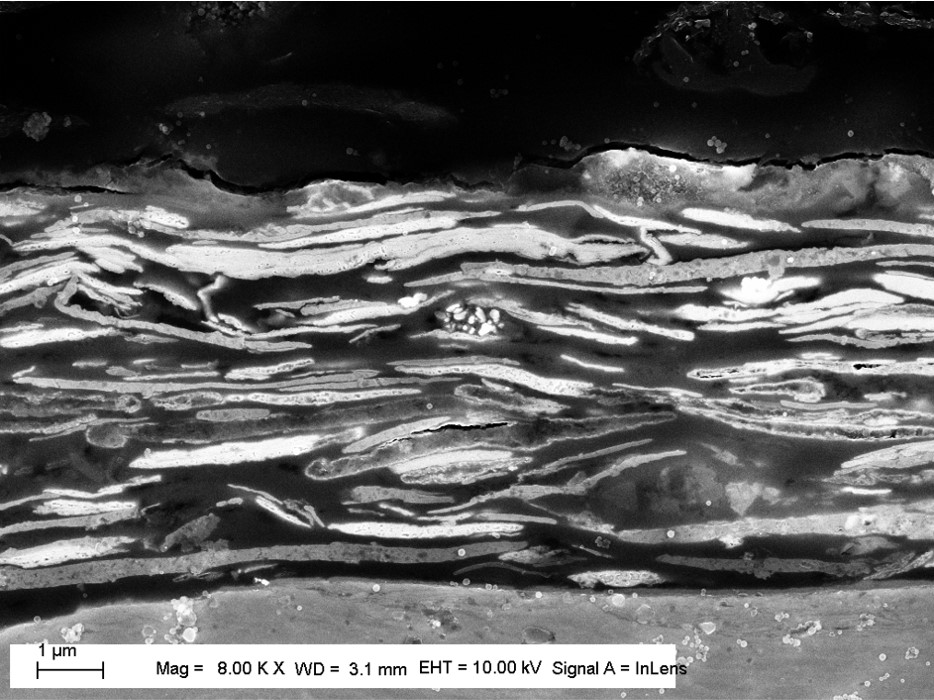
Sacrificial protection
When steel is coated with zinc flake, cathodic protection is achieved. Because of its lower electrochemical potential, compared to iron, zinc oxidizes preferentially: it “sacrifices” itself to protect the steel substrate.
Self Healing
The cathodic protection of zinc produces various kinds of salts (oxides, hydroxides, carbonates…), also known as “white rust”. Thanks to their mobility and adherence properties, these compounds heal the damaged areas of the coating and therefore act as a corrosion inhibitor to protect the substrate.
Adhesion to substrate
Our zinc flake sol-gel binders create strong covalent bonds with the substrate, which ensures excellent adhesion. In addition, at the interface, the migration of corrosion precursors (O2 and H2O) becomes more difficult, which reinforces the barrier effect of the coating.


